Home » Expectant Moms

INTRODUCING
PULSENMORE
Remote & professional assessment of fetal well being for healthier, happier beginnings


Prenatal care from the comfort of your home
We all want to experience pregnancy with peace of mind. Ensuring the well-being of your fetus and fostering a connection with your baby, as well as involving your family in the pregnancy journey, are significant aspects of this period. Frequent clinic visits can be stressful and challenging, especially for those with demanding schedules or living in remote areas. Pulsenmore brings prenatal care into the comfort of your home.
The reassurance you need
Clinically approved and validated
Peace of Mind, control and convenience
Peace of mind and continuity of care during pregnancy

Pulsenmore’s home ultrasound is a safe, reliable, and user-friendly solution that transforms your personal smartphone into a clinically approved and validated home ultrasound device. Prescribed by a physician, the Pulsenmore system includes compatible software that makes scanning quick and simple.
You can opt for a teleultrasound meeting (“clinician-guided”), where a doctor meets you online, guids the scan in real-time and interprets it immediately, or you can scan asynchronously (“App-guided”). In asynchronous scanning, once your scan is complete, the images are automatically sent to a dedicated clinical team for review. The team then assess key parameters that indicate your fetus’s well-being, providing you with the reassurance and continuity of care you need.





How it works
The doctor will provide you with a link for a teleultrasound consultation, offering one of two modes. In the Clinician-Guided scan, the doctor meets you online to observe the scan in real-time, providing immediate diagnosis and guidance. Alternatively, in the App-Guided scan, you perform the scan independently, and the scans are uploaded to the cloud for later review & feedback by the doctor.
1
Download the Pulsenmore Mobile App
2
Connect & pair the Pulsenmore cradle with your smartphone
3
You will be prescribed either App-Guided or Clinician-Guided scan
4
Once you scan, the videos are generated
5
Your scans are reviewed, and you receive clinical diagnosis


Pulsenmore cradle


Pulsenmore application
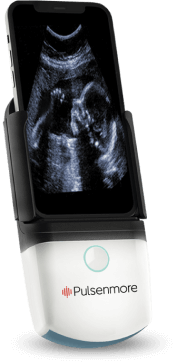

Self-scan ultrasound device



Empowering expectant moms
Pulsenmore empowers you with a home ultrasound solution that allows you to perform basic ultrasound scans from the comfort of your home while receiving remote clinician guidance and diagnosis. With Pulsenmore, you can ensure high-quality prenatal care without the need for frequent clinic visits. Perform ultrasound scans at your convenience and gain access to expert medical interpretation from the comfort of your home.
The device is tailored for use throughout your pregnancy, starting as early as 14 weeks for singleton pregnancies, and is compatible with most smartphones.
Experience the benefits of advanced prenatal care with Pulsenmore, and enjoy a more relaxed and connected pregnancy journey.




The different modes of operations
Clinican-Guided Mode
The real-time teleultrasound consultation allows you to connect with a doctor online. Doctors can guide and control the scans from their dashboard, and provide immediate diagnosis.
App-Guided Mode
Follow a self-scan protocol using the Pulsenmore mobile app. Your scans are automatically uploaded to the clinician dashboard for review and diagnosis
Testimonials
Clinicians & patients love Pulsenmore!


Shir Tzipori Shmuel
Expectant mum
There have been times when I felt the home ultrasound really saved me. After worrying a lot at the start of my pregnancy, using the device gave me the sense of security and calm that I needed.


Shirli Devinkasky
Expectant mum
The home ultrasound is so easy to use. It’s compact and does not require much fuss… I even found a quiet corner in the office to use it if necessary. As a single mother, the device gives me the peace of mind I need.


Doria Wiezman Marely
Expectant mum
After doing a Pulsenmore scan in my 39th week, I got a call from the doctor referring me to the ER with too little amniotic fluid. My little girl was born within less than an hour! Every minute was critical at that point and this really saved us!


Meital Sonenzon
Expectant mum
At 39 weeks I felt my baby’s movements had slowed dow and did a scan. I soon got a call from the Clalit Home Ultrasound Center referring me to the ER because fetal movement wasn’t observed. I was quickly induced and gave birth! Now I believe the Pulsenmore home ultrasound device is a necessity for any pregnancy.


Gal Arditi
Expectant mum
Our pregnancy was considered high risk and that made us more anxious about our baby. When we did home scans with the Pulsenmore device we felt so reassured and grateful for this awesome technology. It spared us at least four emergency room visits. We recommend this to anyone!


Prof. Asnat Walfisch
Head of the Hospital for Women, Beilinson
The device provides vital information to medical teams by quickly and accurately assessing the condition of the fetus.
This grants expectant mothers peace of mind and helps avoid unnecessary visits to clinics or ER.
Additionally, Pulsenmore enhances baby bonding by allowing expectant parents to regularly monitor their baby’s development from home.


Prof. Arnon Wiznitzer
Distinguished expert in OBGYN, Full Professor at Tel Aviv University
This pioneering service is poised to change the way pregnancy monitoring is managed and experienced. Home ultrasound provides crucial information regarding amniotic fluid levels, fetal movements, and its heartbeat. An additional major advantage it offers is an exceptional quick and accurate snapshot of the fetus’s condition at any given moment, thus providing peace of mind.


Dr. Avi Tsur
Director of the Sheba Women's Health Innovation Center, Director of OBGYN
Sheba Beyond have managed to implement a hybrid program for high risk pregnancies by harnessing the power of innovative technologies such as Pulsenmore’s Remote Ultrasound. Now, clinical visits can be seamlessly replaced by virtual consultations, and high-risk pregnancy hospitalizations can be shifted to the comfort of home.


Lior Wolff
Head of Translational Medical Innovation
We are shifting healthcare into the patient’s hands. We are all aware that there is a shortage in many aspects of
medicine – but with the Pulsenmore teleultrasound solution we can turn patients into engaged partners.
Prof. Asnat Walfisch
Head of the Hospital for Women, Beilinson




Prof. Israel Meizner
Chief Medical Officer Pulsenmore, Prof. of OBGYN
By making reliable fetal ultrasound accessible to women at home, Pulsenmore’s device contributes to a better pregnancy experience and substantially elevates the standard of pregnancy care. In the era of telehealth, a pregnant woman can receive the clinical guidance that she needs in the comfort and privacy of home.


Rotem Malihi
OBGYN Sonographer, Clalit Health Services
Pulsenmore’s home US device provides peace of mind during pregnancy, especially for patients with a history of recurrent miscarriages and IUFD.


Dr. Michal Levy
Senior Physician, MFM Unit, Wolfson Medical Center
With a growing volume of clinical observations, I’m seeing that the home US system has real potential to give doctors better control of the prenatal care path.
And for the patient it could significantly reduce anxiety during pregnancy – thanks to continuity of care between visits.
Dr. Avi Tsur
Director of the Sheba Women's Health Innovation Center, Director of OBGYN




Prof. Alfred Abuhamad
Chairman, OBGYN Department, Eastern Virginia Medical School
I have seen that the ultrasound scan with Pulsenmore is simple and easy to perform, and very intuitive for patients. This device may play a significant role in obstetrics and have an impact on prenatal ultrasound.


Prof. Eran Hadar
Director of MFM, Rabin Medical Center
With Pulsenmore’s home ultrasound, doctors and ultrasound specialists can provide the pregnant women with continuous and individualized prenatal care, and the peace of mind that is much needed during pregnancy. During the COVID pandemic, we progressed a decade in one year and at-home ultrasound is a big part of it.




