Yes, the device is reliable and was tested, validated and approved for use in the US, Europe, Australia, Brazil, Switzerland, the UK, Colombia and Israel. Over 200,000 successful scans have been performed since the launch of the technology. These numbers demonstrate that women can confidently scan themselves, as the diagnoisis it self is being done by a trained clinical team. The remote clinical team can accurately identify parameters related to fetal vitality, such as fetal heartbeat, fetal movements, and amniotic fluid volume, based on these scans.
Home » Archives for November 2025
Resource Center
Explore our comprehensive library of clinical publications, abstracts, news, events, newsletters, marketing materials and press releases.
Discover how Pulsenmore is shaping the future of healthcare delivery
Filter By:

- News
We're excited to announce our new partnership with Cetina sp. z o.o., a leading provider of advanced medical technology in Poland.
- Webinars
Dr. Alex Peahl (University of Michigan) and Dr. Avi (Abraham) Tsur, MD (Sheba Medical Center, Tel Hashomer) discuss the shift towards more flexible, personalized, and hybrid hashtag#prenatalcare.
- Webinars
We're absolutely thrilled by the vibrant energy following our recent webinar, a collaborative effort between Women's Health Innovation Series , Sheba Medical Center, Tel Hashomer, and Beilinson - Rabin Medical
- Webinars
Our recent webinar brought together leading experts in maternal-fetal health to explore the complex psychobiological effects of prenatal anxiety on both mother and child...
- Newsletters
The future of prenatal care is hybrid – and it’s already here. We’re excited to share the latest research, clinical milestones, and global recognition that are shaping the next chapter
- Ongoing research
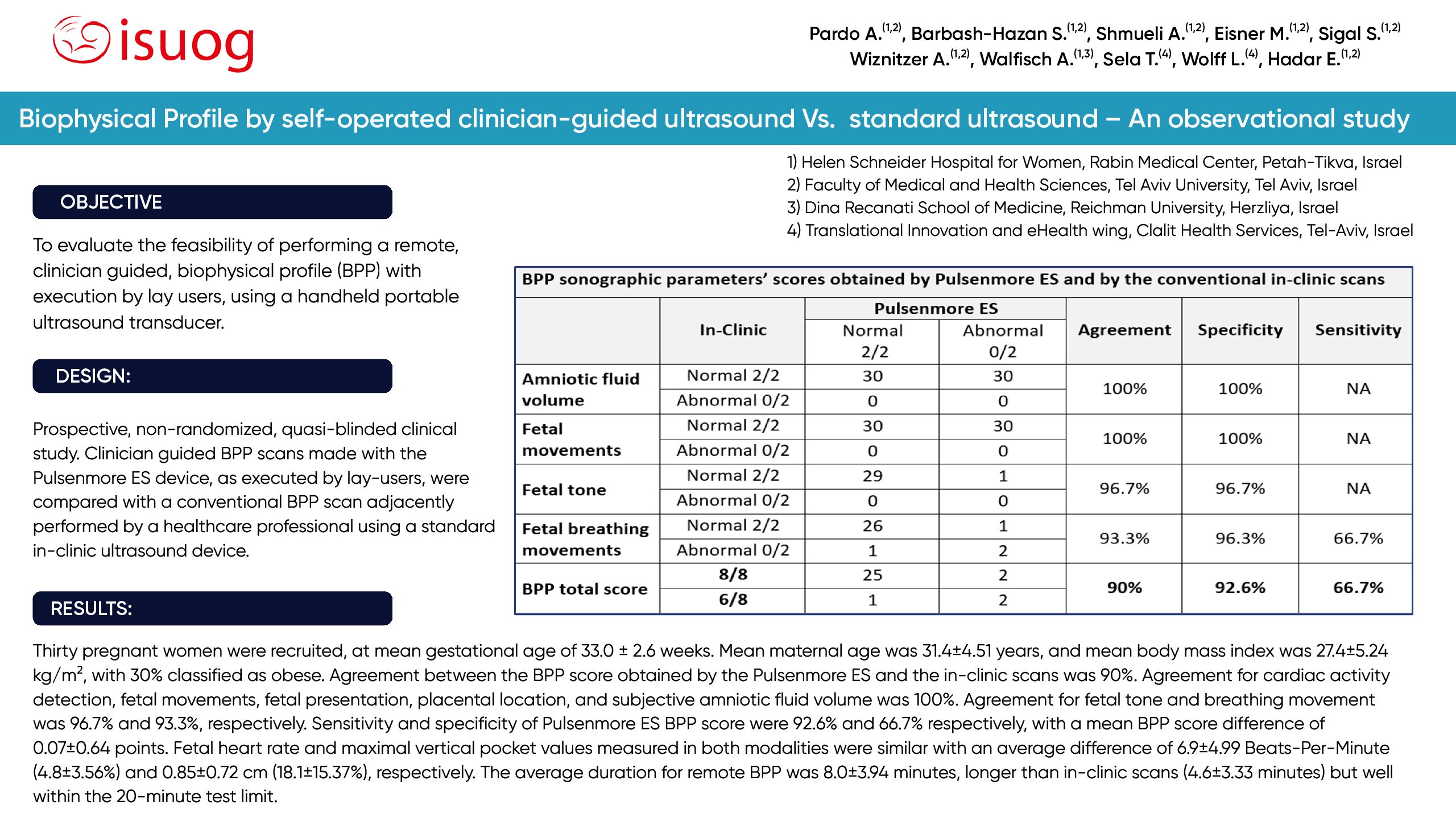
BPP, obtained by the Pulsenmore ES ultrasound device, operated by lay users, during synchronized telehealth guidance, demonstrates high accuracy and reliability for clinical use compared to standard BPP.

the news pulse
We invite you to keep up to date with the latest updates by registering to our quarterly newsletter




FAQ
Patient Frequently asked question
Is the device reliable? How can I scan myself if I’m not a professional sonographer or obstetrician?
How do I know if my smartphone is supported?
The Pulsenmore solution utilizes your smartphone’s processing and video streaming capabilities, communication protocols, display, and power resources. For this reason, not all smartphones meet the compatibility requirements. To insure your smartphone is compatible with the Pulsenmore Home Ultrasound check the Specification webpage.
Why is the device single-paitent use?
Our commitment to patient safety and reliable scans guides the device’s design and functionality. The Pulsenmore device, connecting to a patient’s personal smartphone, optimally utilizes smartphone capabilities, minimizing material and resource consumption for enhanced environmental sustainability.
1. The Pulsenmore self-scan device is designed for use within a specific timeframe and usage limit. Extending its lifetime may compromise reading integrity due to material limitations.
2. Responsibility for device maintenance and home storage after the initial period cannot be assumed, impacting transducer quality and ultrasound scan reliability.
3. The device design and validated cleaning protocol do not allow transfer between patients, to avoid cross contamination and infection.
Can the product be used in a pregnancy with multiple fetuses?
No, the product is subject to use in single pregnancies only.
Can the device increase anxiety or create dependency?
The home ultrasound device is designed to provide continuity during pregnancy care and is intended to give you peace of mind.
Can I scan without medical interpretation?
Every scan requires medical interpretation, and it’s important to wait until the files are fully uploaded. You are not expected to understand or interpret the ultrasound scans; just follow the app’s instructions to scan, and receive a response from skilled ultrasound professionals .