Home » Careers

Meet Racheli
« We’re dedicated to transforming women’s health by making innovative prenatal care accessible to everyone. »
Racheli Sharabi – HR Specialist


Join the Healthcare Revolution
We operate on a global scale , embrace innovation and offer a diverse array of intriguing jobs opportunities that require the ideal individuals to fulfill them
Working at Pulsenmore will give you a great opportunity to learn, grow and contribute to making a positive difference in the future of healthcare


Meet Shmuel
« We care about supporting women through their pregnancy with compassionate care. »
Shmuel Azulay – ES Product Manager









Join the Healthcare Revolution
We operate on a global scale , embrace innovation and offer a diverse array of intriguing jobs opportunities that require the ideal individuals to fulfill them
Working at Pulsenmore will give you a great opportunity to learn, grow and contribute to making a positive difference in the future of healthcare


Meet Oren
« At Pulsenmore, we are passionate about enhancing maternal health and ensuring every woman receives the care she deserves. »
Oren Maman – Director of IT & Cybersecurity









Join the Healthcare Revolution
We operate on a global scale , embrace innovation and offer a diverse array of intriguing jobs opportunities that require the ideal individuals to fulfill them
Working at Pulsenmore will give you a great opportunity to learn, grow and contribute to making a positive difference in the future of healthcare


Meet Rinat
« I love to empower women with technology that supports their health and well-being. »
Rinat Sharon – Israel Marketing Manager









Join the Healthcare Revolution
We operate on a global scale , embrace innovation and offer a diverse array of intriguing jobs opportunities that require the ideal individuals to fulfill them
Working at Pulsenmore will give you a great opportunity to learn, grow and contribute to making a positive difference in the future of healthcare


Meet Ronit
« I am dedicated to advancing healthcare through cutting-edge solutions that improve patient outcomes and enhance quality of life »
Dr. Ronit Shtrichman – Clinical Director









Join the Healthcare Revolution
We operate on a global scale , embrace innovation and offer a diverse array of intriguing jobs opportunities that require the ideal individuals to fulfill them
Working at Pulsenmore will give you a great opportunity to learn, grow and contribute to making a positive difference in the future of healthcare


Meet Roni
« I am passionate about driving technology that empowers individuals worldwide to take control of their well-being. »
Roni Bochman-Ronen – Int. Marcom Director









Join the Healthcare Revolution
We operate on a global scale , embrace innovation and offer a diverse array of intriguing jobs opportunities that require the ideal individuals to fulfill them
Working at Pulsenmore will give you a great opportunity to learn, grow and contribute to making a positive difference in the future of healthcare
Opportunity starts right here
Junior QA Engineer (Temp)
About The Position
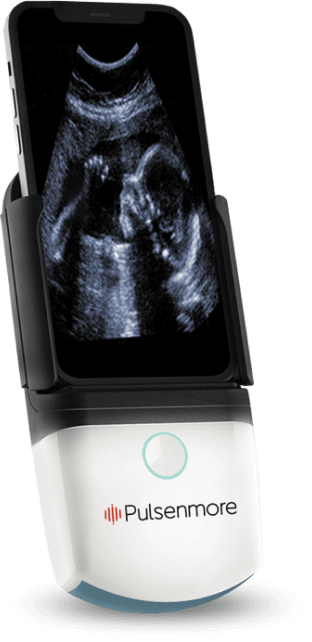
Pulsenmore is a world leader in connected, patient-driven home ultrasound. We are on a mission to improve access to care while increasing convenience and efficiency for patients and healthcare providers alike. Our flagship product, the Pulsenmore ES, is a hand-held ultrasound device that docks with a patient's smartphone. It allows every expectant mother to self-scan and receive a remote clinical assessment from the healthcare provider.
We seek a Junior QA Engineer (Temp). This is an exciting opportunity to join a rapidly growing global Med-Tech company and play a key role in ensuring the quality and reliability of our products.
Responsibilities
Job description:
The QA engineer will part of the QA/RA team at Pulsenmore and will be responsible for:
- Implement and deploy Quality assurance policies and processes.
- Interpret and comply with quality assurance standard and regulations.
- Lead CAPA activities and measure their effectiveness.
- Perform internal audits and manage the audit schedule.
- Participate in external and internal audits and inspections.
- Lead Complaints investigations as Complaint Unit.
- Manage the documentation and record control activities.
- Monitor training and qualification activities.
- Provide inputs to management reviews.
- Support production QA activities (QA release, MRB) as needed.
- Support design control activities as needed.
Requirements
Requirements/Qualification:
Education:
- Practical Engineer/BSc in electrical/mechanical/biomedical engineering
Experience:
- Previous experience in medical device / Quality assurance an advantage
- Previous experience in performing investigations, critical thinking an advantage
- Experience in root cause analysis
Technical skills:
- Language skills, Hebrew, English fluent reading/writing/speaking
- Other languages an advantage
- Computer skills: Office (word, Excel PowerPoint Visio)
Personal skills:
- Team player
- Reliable and accurate
- Good verbal communication
- Works well under pressure
- Attention to details
- Logical thinking
- Fast learner
Qualification requirements:
- QA procedures and Wis related to the responsibility areas
- Internal auditor
- ISO 13485, FDA QSR, MDD, MDR
Apply for this position


PULSENMORE
Bridging Healthcare Gaps with Home Ultrasound
Pulsenmore (TASE:PULS), a pioneer in connected home ultrasound, is on a mission to make accessible, patient-driven healthcare a reality.


Our story
Ultrasound has always been the imaging modality of choice for prenatal care. Traditionally, this meant visits to healthcare facilities, which requires trained professionals and is often costly and requires transportation, childcare, or time off work.
Pulsenmore was born out of a need to alleviate these burdens by offering a fully remote and accessible solution.


What makes us unique?
The Pulsenmore advantage
Our innovative technology enhances resource management by offering patients and caregivers unparalleled flexibility and time savings. We help streamline workflows, improve continuity of prenatal care and amplify overall patient satisfaction.
Practical, safe, and reliable solution for remote sonographic fetal well-being assessment
Prescribed by physicians and complementary to routine diagnostic ultrasound examinations.
Every scan is reviewed and diagnosed by clinicians and can be documented in the patient’s EMR
Better access to care in both rural and urban maternity care deserts

Our vision and mission
Pulsenmore envisions a world where prenatal care is accessible, affordable, and sustainable for all. We aim to revolutionize maternal health by providing remote ultrasound technology that can be used from the comfort of home, transforming traditional prenatal care models and potentially improving outcomes for mothers and babies.